Epigenetic and Immunological Control of Labor Onset
Parturition – the act of giving birth – remains one of the great mysteries in reproductive biology. Not only is the nature of the “clock” that times gestation length completely obscure, but little is known about the sequence of cellular and molecular events initiated at term gestation that ultimately culminate in uterine contraction. Our lack of understanding of such basic features of pregnancy has profound negative consequences for human health, as preterm birth is not only a major cause of neonatal morbidity and mortality but also has sequelae that extend into adulthood. We study mechanisms of parturition onset from two perspectives, epigenetic and immunological. Specifically, we are determining 1) the pathways by which “reversal” of the epigenetic programs established in decidual stromal cells in early gestation contributes to uterine activation in late gestation; 2) the mechanisms by which uterine immune cells become activated in late gestation and in turn themselves contribute to labor onset.
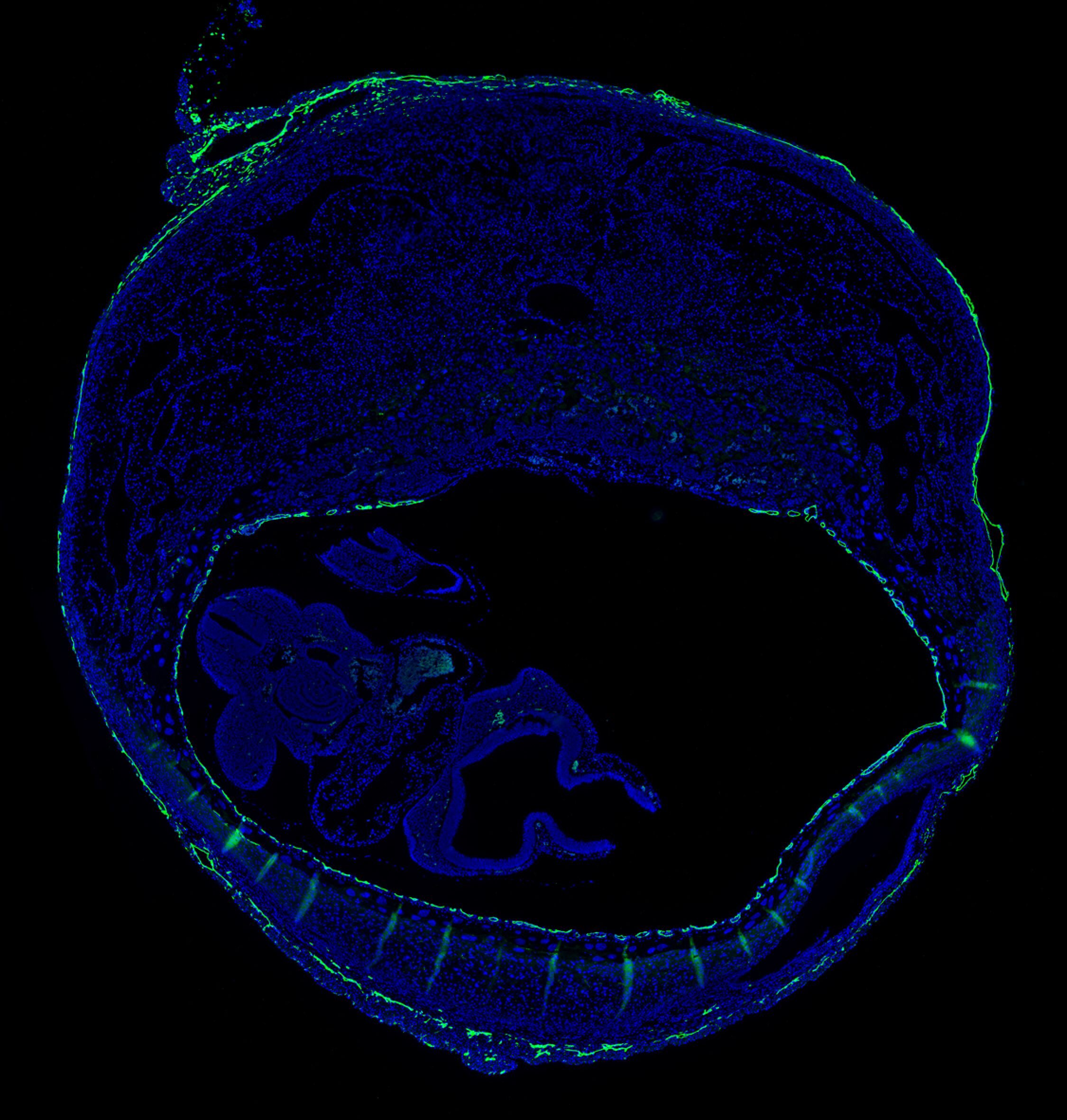
A model for the role of H3K27me3 dynamics in pregnancy and parturition. In early gestation, target gene accrual of H3K27me3 in decidual stromal cells (DSCs) inhibits the expression of genes whose expression would otherwise promote Th1 immunity and myofibroblast-based wound healing within the decidua. Such responses have the potential to cause a variety of pregnancy complications, including preterm labor. In late gestation, genome-wide H3K27 demethylation in DSCs, mediated by KDM6A and KDM6B, leads to target gene re-expression and decidual/fibroblast activation, which in turn generates signals that promote myometrial contractility and labor entry. From Nancy et al., J. Clin. Invest. (2018).

